Home » Laboratories » Study of dust in clean rooms » Pharmaceutical Laboratory – Dust
Pharmaceutical Laboratory – Dust
Pharmaceutical Laboratory - Dust
Year
2023
Customer
IPSEN
Location
France
Typology
Continue navigation :
Our other projects :
Latest news :
Particle concentration and distribution in the production line
EOLIOS Ingénierie studied the concentration and distribution of particles on the production line and in the hall. These studies have allowed us to evaluate whether there is contamination between the two production lines and to develop a system for the aspiration of drug dust.
CFD study of a pharmaceutical cleanroom
Initially, in order to reproduce the phenomena seen by the teams on site, our team of engineers carried out a series of on-site surveys to modelthe equipment as accurately as possible. These readings are crucial for assessing the various parameters and phenomena that can occur within the room.
In order to guarantee an optimized and precise result, our EOLIOSC engineers carried out several repeated surveys to ensure maximum accuracy. Although this data has been collected with great care, there is a certain amount of uncertainty due to the various variations inherent in the human survey. Nevertheless, they can be used to provide accurate airflow trends.
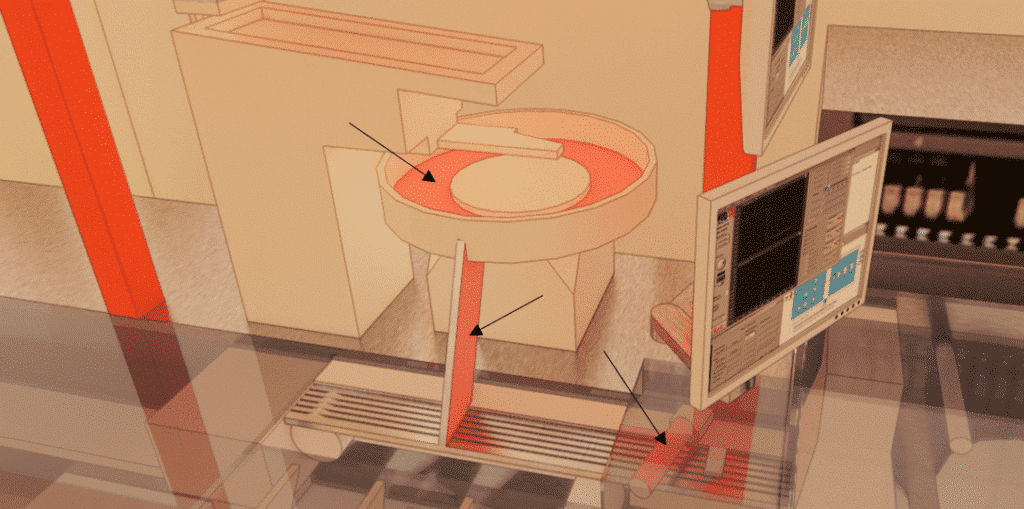
Characteristics of the laboratory studied
The cleanroom where production takes place is an 832m3 room. There are 4 openings. The air is blown through 8 ceiling-mounted nozzles. Recovery is via 16 nozzles located at the back of the room.
Series of on-site measurements
The production line is made up of different modules that enable the product to be canned. Each element has been modeled to scale to provide maximum precision.
Elements external to the production line, but acting as an aeraulic mask and potentially influencing air movement, were also identified for scale modeling.
Creation of a digital study model
The aim of these tests is to evaluate the overall air movements in the production room, in order to identify certain specific phenomena. These phenomena are essentially air recirculation, outgoing or incoming leakage from the production line, and particular air movements between the supply and return air at the back of the room.
As part of this audit, our team carried out a series of tests.
- Initially, the aim of the tests was to evaluate the evolution of air flow between blowing and recovery between the different production lines.
- Secondly, tests were carried out to assess leakage rates from the inside to the outside of production lines, with the aim of highlighting areas of fine dust leakage.
In addition, our thermal and aeraulic engineers were able to take a series of air velocity measurements at various points in the room, with the aim of comparing the results of the simulations with the conditions found on site.
Laboratory audit
Test protocol
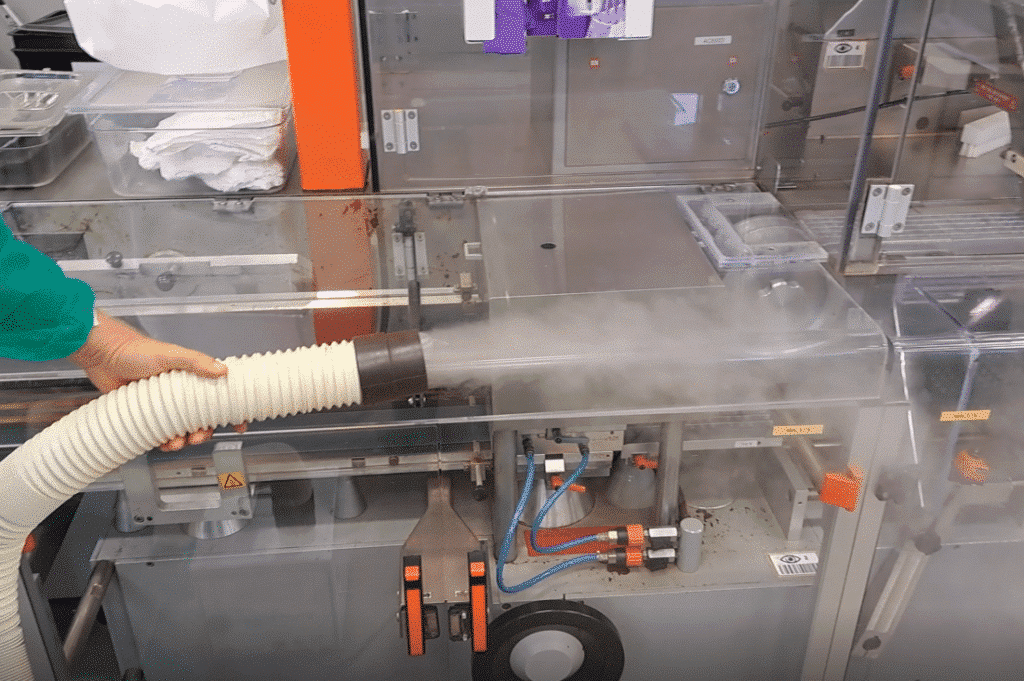
During the tests, the outlet of the smoke machine was pointed in the direction of the flow, and it was then necessary to wait a few seconds for the flow to stabilize, in order to capture the various phenomena as accurately as possible.
We note that the air in the circulation area does tend to flow in a laminar direction to the take-up points. However, the smoke tests did reveal some interesting phenomena.
In fact, the smoke tends to enter through the gap between the partitions separating the two production lines, rather than heading for the nearest rework.
Simulation of the wind around the building
Trends in air leakage from production lines
As a first step, in order to assess majority air leakage from the production line, we positioned the smoke machine in the passenger compartment of the pill pouring area, then filled it with smoke.
After filling, it is easy to distinguish the areas with the highest leakage. These areas are mainly around the door and brush.
The role of CFD simulation
The aim of the study is to estimate the concentration and distribution of fine particles in the production line and in the hall. The purpose of this estimate is to assess whether there is contamination between the two production lines.
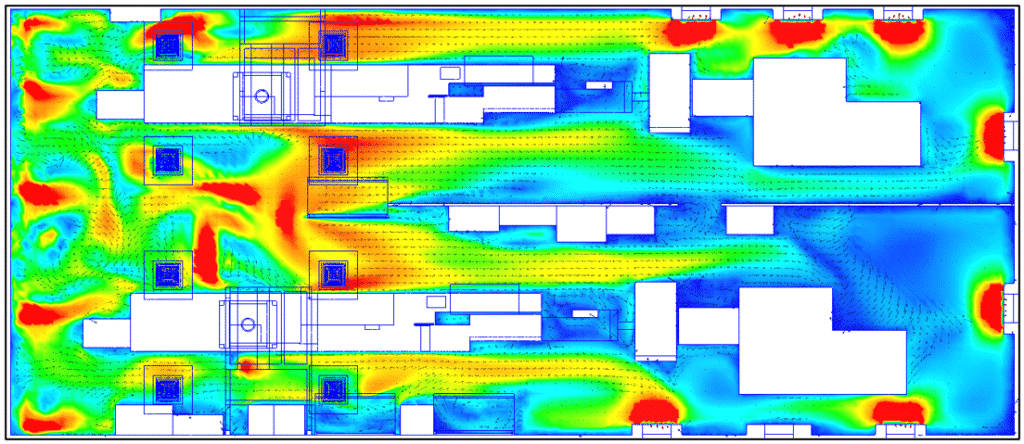
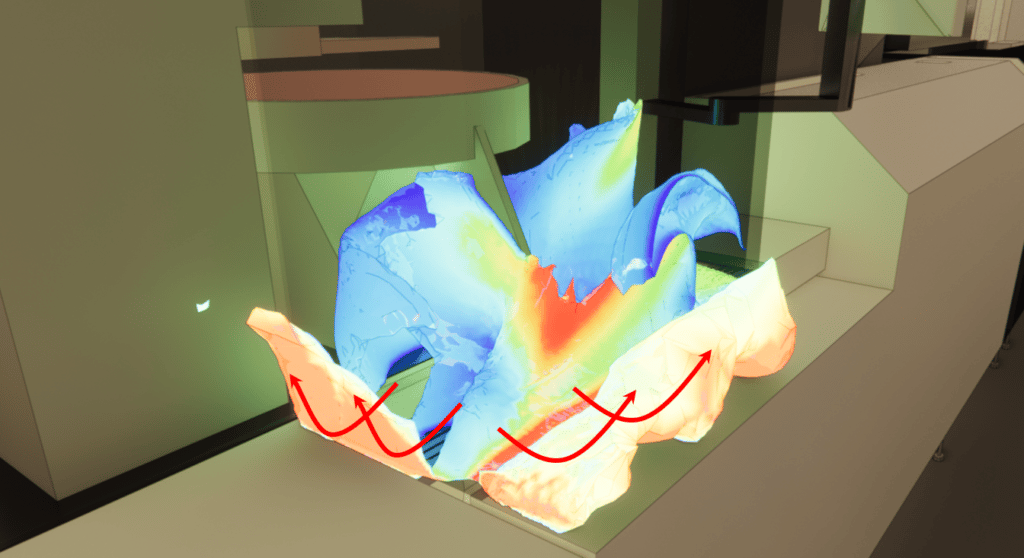
These velocity maps highlight the overall airflow dynamics in the production hall. This dynamic translates into higher air velocity zones on the left-hand side of the room, where air is blown in, and lower air velocity zones on the right-hand side, where air is blown out.
The simulation also highlights phenomena identified during the smoke study.
By comparing the observations made during the on-site smoke test with the simulation results, certain similarities can be observed. The low-speed recirculation zone is located in the opening between the two partitions.
Overall velocities in the room are of the order of 0.2 m/s, with low-velocity zones behind equipment forming aeraulic masks.
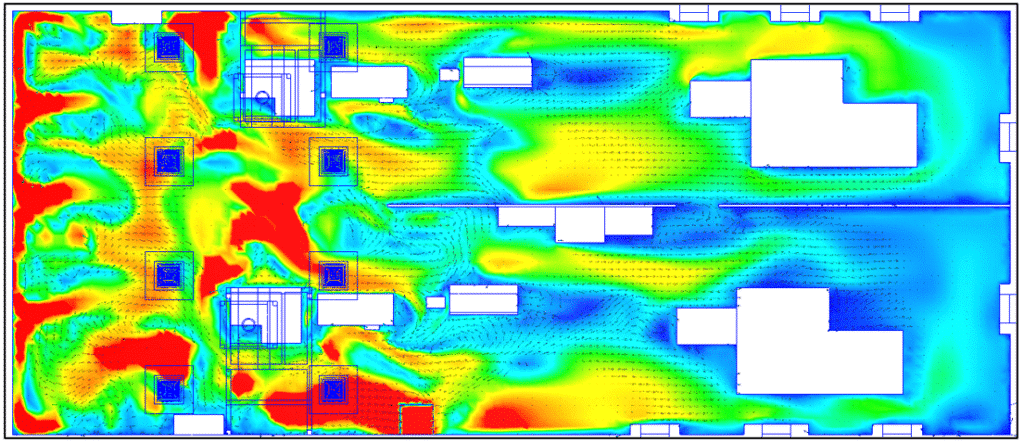
Fine particle concentration study
Dust emission zone
Dust emission zones were calibrated according to the observations of on-site technicians.
There are 3 main zones:
- The tank
- The rail filler arm
- The sorting brush
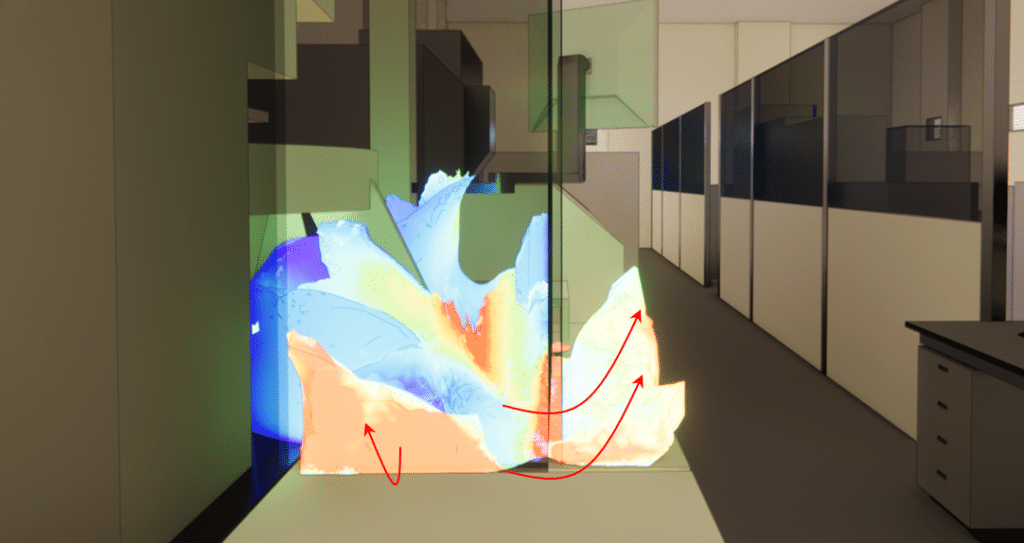
CFD tracing of dust from the machine to the hall
Two main areas of dust leakage to the outside of the machine. These very localized leaks occur through slight openings in doors or gaps between modules. Despite the low proportion of particle leaks, this represents a contamination risk.
The risk of direct contamination between the two machines is unlikely, but unintentional contamination by technicians during maintenance work cannot be ruled out, given the level of dust emitted around the machine.
In order to overcome this problem, we dimensioned extraction systems at the identified emission zones.
CFD simulation for laboratories
Digital simulation offers new perspectives for design offices. This makes it possible to foresee a large number of scenarios and consequently to control all the unforeseen events linked to a bad design. In the case of production plants, multiphysics modeling enables us to take into account all the phenomena at the origin of thermo-aeraulic flows that occur along the production line, from overheating to employee comfort, via a guarantee of non-contamination of production.
Thanks to its calculation servers, EOLIOS models can be simulated in their entirety with great precision in a very short space of time. In addition, EOLIOS’ experience in general aeraulics enables our team to propose innovative and relevant solutions in the case of aeraulics, air conditioning and fine particle propagation problems. Nevertheless, implementing CFD simulations in your design process means calling on experts in fluid mechanics, thermal and numerical simulations to ensure that no problems occur in the future.
Our EOLIOS engineers have extensive auditing experience, bringing their expertise directly on site to optimize the resolution of various problems. In this case, the audit was carried out over the entire production hall, enabling air leaks at machine level to be effectively located, air flows to be studied and production purity to be ensured.
Their cutting-edge equipment enables direct and distinct measurements, guaranteeing an assessment of the site, equipment and materials, and if necessary, a thermal expertise including thermal bridges, heat loss and loss of performance of air-conditioning systems. The various distribution facilities were analysed to identify possible contamination of the production chain.
Smoke tests to check the installation
EOLIOS recently carried out a smoke audit to ensure optimal sizing of the suction systems used in the drug production machine. This audit was crucial to ensure that the extraction equipment was capable of providing effective extraction of the fine dust produced during the production process.
The main objective of this audit was to verify whether the current suction systems were adequate to meet the requirements of drug production. To achieve this, EOLIOS carried out exhaustive tests using smoke agents adapted to the machine’s working environment.
Smoke tests to check installation on acceptance
Thanks to the use of these smoke generators, we were able to visualize and measure the flow of smoke generated by the production machine. These data were then analyzed by EOLIOS engineering experts to determine whether the existing extraction systems were capable of effectively capturing and removing these fumes.
The results of the audit showed that the current extraction systems were indeed correctly dimensioned and could effectively manage the fumes produced by the machine. This confirmation is essential to guarantee the quality of the medicines produced, avoiding any potential contamination due to the presence of toxic or undesirable fumes.
In conclusion, the smoke audit carried out by EOLIOS has played a crucial role in ensuring the performance of suction systems used in drug production. Thanks to this in-depth analysis, we were able to confirm that the equipment was correctly sized.
Continue on this topic
Video summary of the study
Discover other projects
Contamination study – Pharmaceutical production lines
Dust dispersion study – Geology laboratory
HVAC – Medical equipment warehouse
VOC treatment process improvement
Cleanroom aeraulics
Pharmaceutical Laboratory – Dust
Qualification of fume cupboards – Laboratory
Study of a clean room